The FDA just made history. They approved the first CRISPR (Clustered regularly interspaced short palindromic repeat, a genetic manipulation system) technique to treat a human disease.
The CRISPR device was invented by a team from MIT or UC Berkeley. (While they have been each claiming the invention; the team from Berkeley- Drs. Jennifer Dudan and Emmanuelle Charpentier- were awarded the Nobel Prize for the development of this system.)
(I am not going to fully explain how this process works. Instead, I will provide a short explanation. If you are interested, you can research the concept further. This genome editing technology can modify genes in living cells; it can create diagnostic devices or repair mutations at precise locations in the human genome. A CRISPR sequence is transcribed into a short RNA sequence that can then be matched to the DNA structure. It has been called the Swiss Army Knife for genetic manipulation, a molecular scalper, a copy-and-paste technology for DNA editing.)
The new system approved by the FDA called Casgevy (Vertex Pharmaceuticals) has already been approved for use in the UK. Now, it will be available here in the states. The disease to be treated is sickle-cell, one that affects about 100K folks of African ancestry in the USA, due to a faulty hemoglobin molecule.
The sickle cell genetic mutation changes the shape of hemoglobin from a flexible molecule to a rigid crescent or sickle shape- much less prone to pick up and carry oxygen. The mutation can block blood flow, create organ damage and stroke- and leave the patient in pain. This makes for deficient oxygen transport in the body.
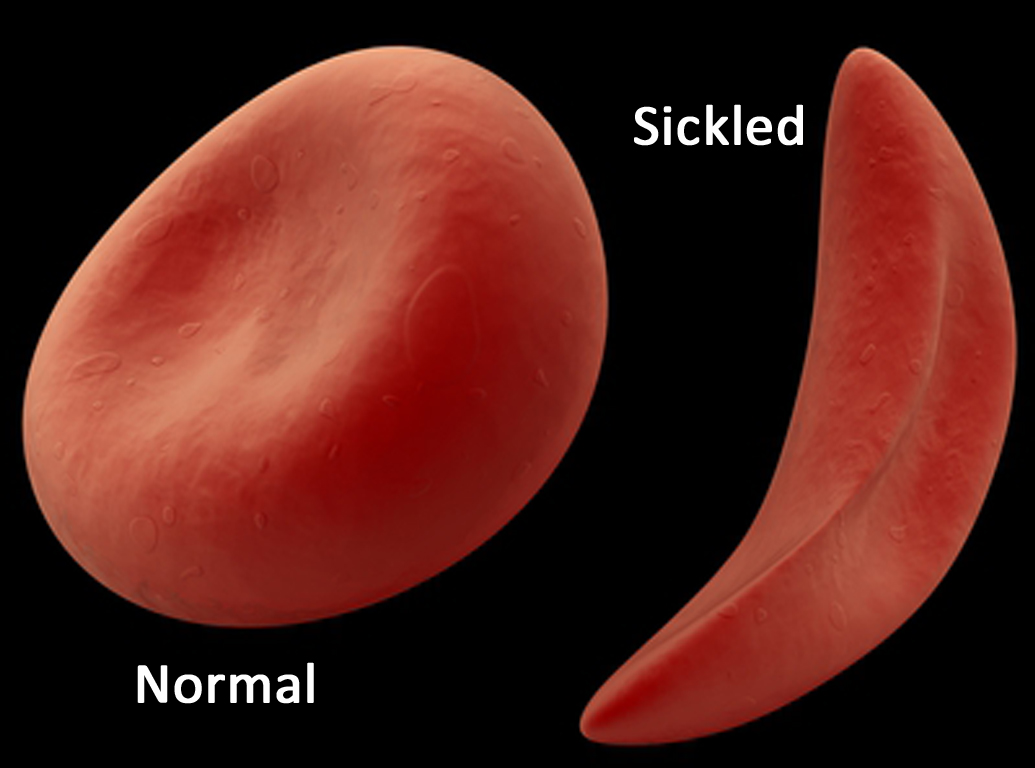
Note that this device does NOT deal with the faulty hemoglobin; instead, it activates a form of fetal hemoglobin found in the body. And it actually affects the gene that inhibits the production of fetal hemoglobin in adults- so more fetal hemoglobin is produced.
An aliquot of the patient’s blood is removed, purified, to which a CRISPR protein, targeting the BCL11A gene. Once the cell editing is complete, the fluids are returned).
Up until now, the treatment was a bone marrow transplant. This requires testing many folks, hoping to find the (near) perfect match. However, this new CRISPR therapy is not going to be used in every medical center, due to the needs of specialized equipment and the price (more than $ 2 million)- which may or may not be covered by insurance. Yes, we can expect a slow adoption of the new therapy.
Nevertheless, this should open the floodgates for the adoption of many more CRISPR repairs of the human genome.







