Please note: I meant to post this before Friday’s post, because of its ramifications. That’s the problem with having many items in the queue. I can forget which posts correlate with others…
My friend knows that I detest a certain commercial. The music is putrid, the narrator is whiny- but hopeful. That ad is for Cymbalta. An anti-depressant that has recently been recast for another disease, that is as well treated by drugs as depression, fibromyalgia. It is one of the SSRI [Selective Serotonin Reuptake Inhibitor] (Cymbalta is also an SNRI- [norepinephrine is the N]) that have the same m.o. (modus operundi, or method of operation).
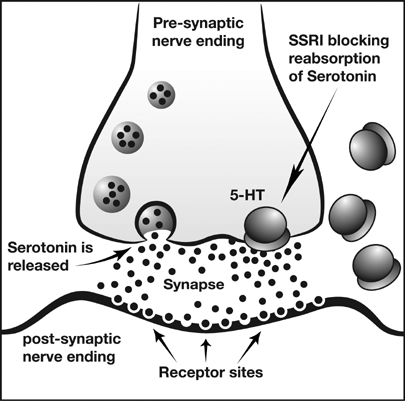
Hope is the ultimate anti-depressant; with hope, depression is gone. Psychiatrists know that employing hope and imparting it to their patients is the best therapy of all. And, that’s what the pharmaceutical companies have been trying to sell with all their anti-depressant ads, as well. Garner hope, restore your vitality, banish depression.
Now, another revelation. The FDA only requires two (or maybe three) trials that demonstrate that the proposed new psycho-active drug makes the patient feel better than a placebo (a similar shaped tablet that has no active ingredients). Read that again. You see, in this market segment, the FDA does not require the drug company to have all its tests and trials evaluated- just the two or three for which it is using to request approval. Perhaps there were a million tests, but two worked the way they wanted. (That million is hyperbole, but you get the idea.) Yup- you guessed it, the drug could be approvable.
Enter Irving Kirsch. Professor of Psychology, University of Hull (UK). Along with a Canadian and a few American profs- Drs. Deacon, Heudo-Medina, Scoboria, Moore, and Johnson. What did they do? They used FOIA (the Freedom of Information Act) to obtain the information used to evaluate all the anti-depressants approved from 1987 to 1999: Prozac, Paxil, Zoloft, Celexa, Serzone, and Effexor.
What did they find? 47 clinical trials performed for these six drugs. And, for 35 of them, there was sufficient data to compare multiple independent studies. Drum roll, please…. The data demonstrated that these drugs were really no better than a placebo.
This analysis was published in PLoS, the online journal of science; the title indicates one of the problems: Initial Severity and Antidepressant Benefits: A Meta-Analysis of Data Submitted to the Food and Drug Administration. A “meta-analysis” analyzes the results of two or more studies, which must be sufficiently similar, so that one can combine and compare the results. Statistically, this affords one the ability to identify the common effect(s). The weakness of a meta-analysis is that it is not hard-science, since sources of bias in the original studies cannot be controlled; badly designed studies yield bad statistics; and the published studies used may have exaggerated outcomes (no one publishes studies with no significant results). [That means that while these results may seem significant, one must examine the original studies carefully.]
Some researchers have responded to this analysis averring the data demonstrates that some anti-depressants have a small positive effect for many, with a larger effect for a few- a meta-analysis would be difficult to discern such phenomena. (A previous, less inclusive, study did demonstrate that there was a marginal clinical benefit- the SSRI’s improved the HRSD (Hamilton Rating Score for Depression) score by 1.8 points (FDA) to 3 points (NICE, the UK equivalent); a score of 11 (out of 42) is considered to be an indication of depression.)
But, the reality is that the drugs don’t really make the difference to the patient- the psychologist, the psychiatrist, the trained professional yields the major difference. And, they should work with the patient until some cocktail is found that provides the desired relief (or try to resolve the problem without drugs).
[Note: Dr. Kirsch wrote a popular book: The Emperor’s New Drugs: Exploding the Anti-Depressant Myth, Basic Books on this matter.] You might want to click on the link and get the book for yourself.








Very interesting information – it either shows the power of the mind to heal or lack of efficacy for most drugs.
I think it shows both, Tor.
But, part of the problem is that affording a drug approval, when it changes a score by 2 or 3 points out of a 44 point scale, means the bar is too low. It would be my guess that any two practitioners would evaluate the same person within such a numerical difference. Could you imagine having a patient take a $ 300 drug (monthly cost) to treat his HIV that changed the titer of antibodies by 1%?
Thanks for dropping in… Wait till the next discussion about another drug (also in the queue).
Roy
Hey Roy,
Sometimes, the patients have in their “heads” that unless and until they are popping pills, nothing might work out or get better. Let’s say, for a psychological problem it is very common that most of them are accompanied by physiological symptoms. For example, panic attacks are accompanied by shortness of breath, palpitation, uneasiness and sweating (though these may differ from person to person). Now what drugs does is work on the physiological causes, reduce the signs of sweating, the breathing and the likes; the problem hasn’t gone. Only it’s physiological manifestations have been reduced / worked on. The shrink / psychiatrist works on getting to the root cause of it. So that you as a patient, get rid of the problem and thus, the physiological discomfort.
Though drug use for severe cases is mandatory and has provided relief, there has to be difference between taking drugs for relief and drug dependence.
Loved the post; understood it well 😉
Hope you have a nice day!
Hajra:
This area is one that is fraught with possibilities. And, I don’t want to be the one to be the arbiter, either. I know many folks who have problems and need help. My issue is when someone is classified as depressed if they fall 11 on a scale of 44 (which I would term unhappy or troubled- but not depressed). If they drop to 10 on these drugs, they’ve been helped. But, it would be just as easy to have another clinician evaluate them and have the same results obtained SANS medication.
I would vote for a score of 25 or so for the diagnosis. But, more importantly than that, I would expect a drug to provide at least a change in score of 6 or so- one that would remove statistical variation from the evaluation.
Roy